ABSTRACT
The quality of the homoeopathic medicines depends on the production and collection of raw materials. Homoeopathic pharmacopoeias throughout the world prescribe microscopy, physicochemical, instrumental, chromatography and biological analysis for standardization of homoeopathic medicines. Despite the growing use of homoeopathic medicines worldwide, few of the WHO Member States regulate these medicines. It is usually taken for granted that the safety of homoeopathic medicines should not be a major concern as these medicines are often highly diluted when administered. However, this is not always the case. Moreover, the variety of materials used (medicinal plants, animal and human materials, pathogens as well as minerals and chemicals) and other technical aspects of the production and manufacture of homoeopathic medicines may constitute potential risks to their safety. GMP (Good Manufacturing Practices) are a part of quality assurance aimed at ensuring that products are consistently manufactured to quality appropriate to their intended use2.
KEYWORDS: quality, quality control, Homoeopathic pharmacy, manufacturing process, Good Manufacturing Practices, Organon of Medicine, Good Agricultural Practices, Good Harvesting Practices, Good Laboratory Practices.
INTRODUCTION
“A dedicated physician can only be sure about the healing properties of a drug when it is made as pure and as perfect as possible.” – DR. HAHNEMANN11
Quality is the status of the drug that is determined by identity, purity, content and other chemical, physical or biological properties1.
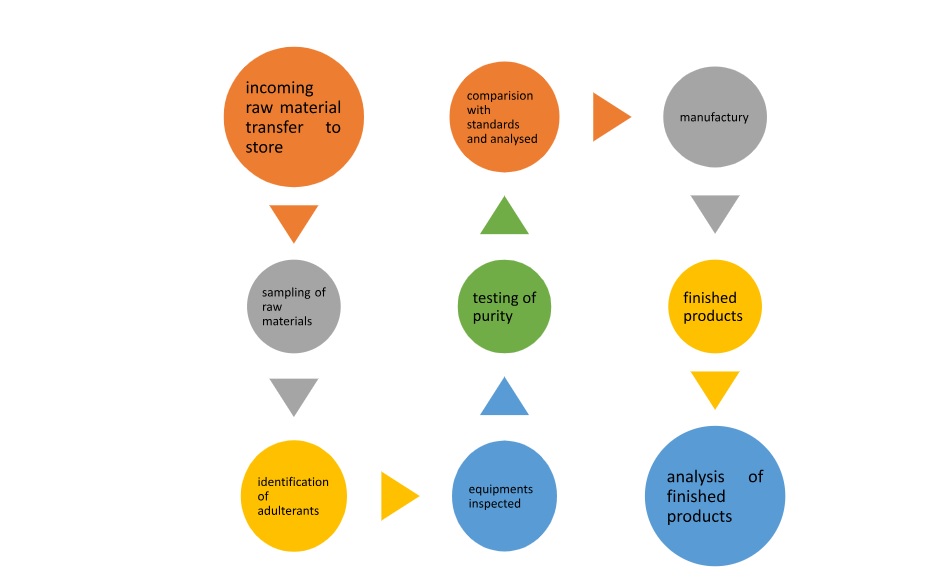
Quality control is referred to the processes involved in maintaining the quality and validity of a manufactured product1. The quality should be tested right from the receipt of raw materials till the final packaging. It includes quality specifications, correct ingredients in correct proportions, up to date purity, processed as specified in different pharmacopoeias, packaged in a proper container, proper labelling and storage for maintaining the proper quality1,6. Prime factors that play a vital role in quality control are materials, personnel, equipment’s, environments, methods and infrastructure1.
In India the Homoeopathic Pharmacopoeia Laboratory [HPL], Ghaziabad, 1975, under the ministry of Health and Family Welfare, Government of India is responsible for Quality control & Standardization of Homoeopathic Drugs1.
NEED OF QUALITY CONTROL
In today’s era adulteration of any product is burning issue. Quality-assured, safe and effective medicines are a pre-requisite for high-quality health care as the incidence of substandard or falsified medicines is growing. In Homoeopathy quality of medicine is a vital factor3.
LITERATURE REVIEW
Schematic representation of Quality control1
Homoeopathy and Homoeopathic Medicines:
The central tenet of homoeopathy is that “like cures like” (in Latin: similia similibus curentur), in a holistic approach to the totality of the patient’s symptoms2,9. Homeopathic medicines are based on the principle that high dilutions of potentially active molecules retain a memory of the original substance. Hence, the starting materials, mother tinctures are subjected to a process of serial dilution and succussion. Dr. Hahnemann employed this process of potentization to diminish the toxicity of potentially hazardous substances.
From the safety point of view, it is important to note first that, although homoeopathic treatments often utilize ultramolecular dilutions of the starting material (above Avogadro’s number), there are also homoeopathic medicines of considerably lower dilution which do contain molecules that may be active in the biochemical sense. Although homoeopathic medicines are in general considered to be safe when administered appropriately, toxicological aspects should not be neglected especially when using lower dilutions2.
Dr. Hahnemann, the father of Homoeopathy has also written in the 6th edition of Organon of Medicine about the quality control in the following aphorisms5
§123 – Unadulterated herbs
§264 – Genuine medicines of unimpaired strength
§265 – Employment of correct medicine prepared by physician himself
§266 – Substance belonging to the animal & vegetable kingdoms possess their medicinal qualities most perfectly in their raw states
§267 – Medicines: indigenous plants
§268 – Quality control to check genuinity of herb
§270 – Pure quality of alcohol & globules4,5
POTENTIAL SAFETY ISSUES
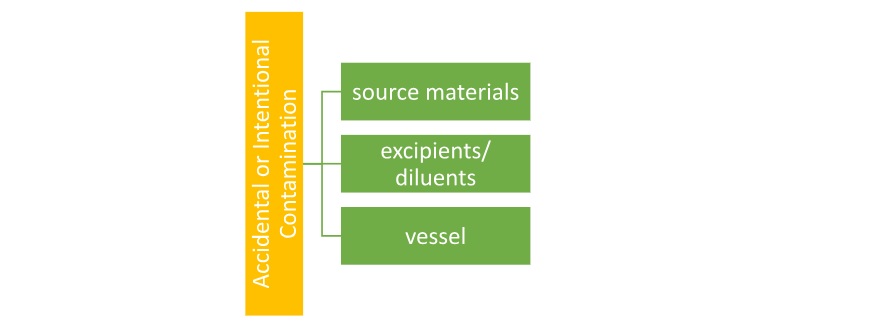
Two main potential hazards are the one which is related to the source material and the other is related to the procedure that is used for manufacture of the finished product2.
Homoeopathic medicines may employ material from problematic sources, the use of which is restricted in conventional medicine. Nosodes – comprise dilutions of pathogenic organs or tissues, causative agents such as bacteria, fungi, ova, parasites, virus particles, and yeast; disease products; Sarcodes – excretions or secretions. All materials of animal or human origin are at risk of containing pathogenic agents. Homoeopathic medicines may be based on toxic source materials from animals or plants, while others, particularly in their fresh form are prone to degradation processes or microbiological contamination2.
Good manufacturing practice (GMP) guidelines covering the manufacturing process, premises, personnel, packaging and labelling apply to homoeopathic medicines as well as to conventional pharmaceuticals. Failure in applying GMP may lead to major quality and safety concerns such as misidentification, impurity of starting material, cross-contamination or incidental contamination2.
The unique characteristics of the manufacturing of homeopathic medicines has a number of specific implications and demand specially qualified and experienced personnel2. These have to handle toxic materials, fresh ones, that are prone to degradation processes and microbial contamination and that are derived from animals or human sources2.
QUALITY CONTROL ISSUES FOR HOMOEOPATHIC MEDICINES
It is mandatory to follow the guidelines given by Good Agricultural Practices [GAP], Good Harvesting Practices [GHP], Good Laboratory Practices [GLP]1.
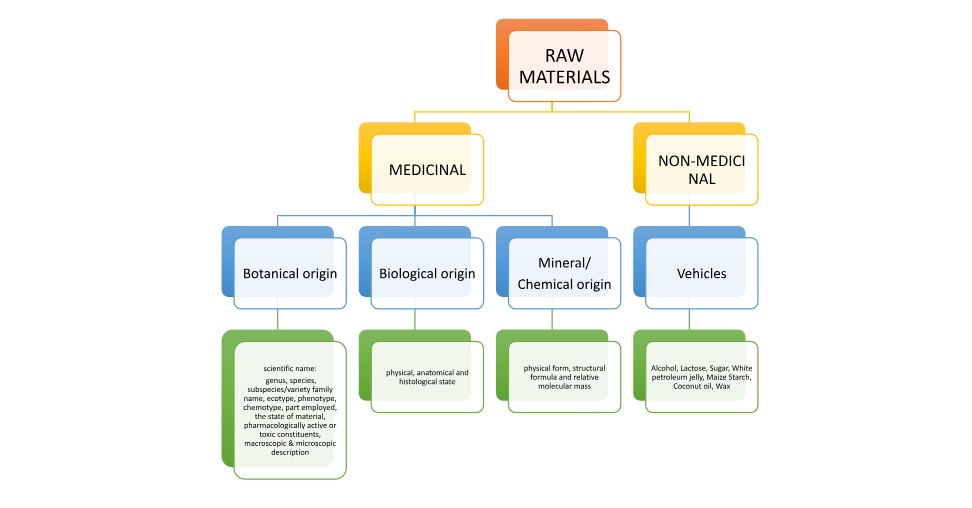
PLANT MATERIAL
Due to the complex and variable nature of plant material and its possible contamination with microbes, insects, pesticides, heavy metals, fumigants, mycotoxins and radioactivity, adequate control of source material, storage and processing assume particular importance when plant materials are used in the manufacture of homoeopathic medicines2.
Manufacturers are called to follow exemplary standards and provisions regarding identification of source material, limit tests, and complementary tests2.
The following characteristics should be justified:
- parts/material of plant used; macroscopic description of plant and plant material
- microscopic characteristics
- identity tests
- purity tests
- moisture/water content
- determination of content of toxic constituents
- method for preparation of mother tincture2
ANIMAL MATERIAL
They raise concern about microbiological and viral contamination2.
Adequate and validated procedures such as filtering, pasteurization, sterilization or precipitation have to be used depending on the individual raw material, contamination or pathogenic load, especially to establish the first safe preparation2.
The description of the following is needed:
- identification of the source of the animal material, including information on whether animals were bred or caught in the wild and the place of breeding or capture in the wild
- parts/material of animal use
- anatomical description
- histological description
- identity tests
- purity tests
- moisture/water content
- determination of content of toxic constituents
- method for preparation of the initial homeopathic preparation2
HUMAN MATERIAL
Tissues, blood or fluids are a potential source of infection through a variety of known and unknown transmissible agents. Whenever any blood product is to be used, or contamination of blood is possible. Since human-derived materials such as pituitary or liver extracts, albumin, or tonsils may be contaminated by bacteria, virus or quality control measures should address these issues2.
MINERALS/ CHEMICALS
Analytical tests should be carried out to determine the identity and the source or origin, possible contamination with heavy metals and any other possible toxic constituents2.
The following are the requisites for the first dilution/trituration:
- method of preparation
- description and characteristics
- identity tests
- purity tests
- determination of content
- determination of toxic constituents2
MOTHER TINCTURES
They should comply with pharmacopeial specifications and quality requirements in official use, or those of other officially recognized documents.
The following are to be justified:
- method of preparation
- appearance and description
- identity tests
- purity tests
- procedure for stability tests
- determination of content
- determination of toxic constituents2
FINISHED PRODUCT
They should be tested for:
- identity and content
- quality of dosage form
- residual solvents, reagents or incidental contamination
- stability2
FOR EVALUATION OF DRUGS THE FOLLOWING METHODS ARE UNDERTAKEN7
ORGANOLEPTIC EVALUATION
Evaluation of drugs with the aid of human senses. It includes macroscopic appearance of drugs.
- Shape and size – The parts available in the market are of different sizes or broken into pieces; example – conical shape: Aconitum. Sizes are prescribed in length/ breadth/ diameter (cm/ mm)
- Fracture – to study how plant breaks when subjected to requisite pressure
- External markings – example: furrows, wrinkles, ridges etc of various types
- External colour – example: white, yellow, grey, ash etc
- Flowers and leaves – botanical terms are used for identification
- Odor – the characteristic Odors like aromatic, balsamic, camphoraceous, spicy etc indicate comparison with other substances in nature.
- Taste – pungent, bitter
- Colour – standardized according to Inter Society Colour Council, National Bureau of Standard Method7.
MICROSCOPIC EVALUATION
- Isolation of drug constituents: Chemical solvents, Micro-sublimation
- Identification of drug constituents: crystallography, melting point determination, confirmative tests7.
BIOLOGICAL EVALUATION
By applying bio-assay (pharmacological activity) we can evaluate or standardize many drugs7.
CHEMICAL EVALUATION
Chemical evaluation covers isolation, identification, purification and determination of characteristics. They help to determine the identity of drugs and their adulterations.
Chemical assay includes
- Colour reaction test
- Molisch and Bradford’s test
- Determination of acid value
- Determination of iodine value
- Determination of saponification value of fixed oils
- Determination of acid-insoluble ash
- Determination of water-soluble ash
- Determination of sulphated ash value7.
PHYSICAL EVALUATION
- Fluorescence test – solubilities, specific gravity, refractive index, melting point, congealing point, optical rotation, water content or loss on drying
- Chromatographic study of drugs – column or adsorption chromatography, paper chromatography, thin-layer chromatography, gas chromatography
- Counter-current methods and electrophoretic methods of analysis7
PACKAGING AND LABELLING REQUIREMENTS FOR THE SAFE AND PROPER USE OF HOMOEOPATHIC MEDICINES1,2
Packaging is the art and science of, and the operations involved in preparing articles for transport, storage and use. The stability of the product is totally dependent on the proper functioning of the package7.
- name and address of manufacturer, packager or distributor
- manufacturer’s batch number
- registration number
- net amount of the product in the container
- common name of dosage form, the traditional homoeopathic name commonly used in the geographical area
- statement that identifies the product as homoeopathic – e.g. “homoeopathic medicine” or “homoeopathic medicine for anthroposophic use”
- scientific name of the active substance or the traditional homoeopathic name of the active substance, as given in recognized pharmacopoeias in official use, the degree of dilution/potency and a reference to the pharmacopoeia that was used for the method of preparation
- quantity of the active substance in the dosage form
- excipients
- directions for use and dosage requirements
- indications
- storage conditions
- warnings about alcohol or lactose
- warning that advises the user to consult a doctor or qualified health care professional if the symptoms persist or worsen
- route of administration
- expiry date2
- The containers must be well-closed, air-tight and hermetically sealed.
- Traditionally, glass is the most widely used container and it must be strong enough to withstand the physical shock of handling and pressure.
- Metals are not used as packaging material because of their reactiveness, high cost and weight.
- Paper may be used to dispense unit powder medicines7.
- Manufacturing License Number or ‘Mfg. Lic. No.’ is the license number of the manufacturer and indicates that the authority granting license is satisfied with the conditions and that it is competent in manufacturing homoeopathic medicines10.
- Homoeopathic medicine having more than 12% alcohol shall be packed in 30ml only
- For hospital/dispensaries 100ml packing is allowed1
The above-mentioned guidelines are also mentioned in the RULE-106A & RULE-106B, The Drugs and Cosmetics Rules, 19451,8
HAZARDS ENCOUNTERED BY THE PACKAGE
Package and its contents pass through a number of stages and is encountered by various hazards like shock, compression, vibration; in the case of medicinal fluid which has already been sufficiently potentized, receives an enormous number of additional succussions during the transport and on their arrival, they are scarcely fit for use, at least not for susceptible patients on account of their excessive strength7.
DISCUSSION & CONCLUSION
In order to prevent the spread of substandard drugs and to ensure that the drugs manufactured or sold or distributed throughout the state are of standard quality, the drug regulatory mechanism should be strengthened at the state level for the improvement of the quality of drugs used in Homoeopathy5. As a conclusion one can state that the Homoeopathic preparations with the help of Quality Control have a therapeutic relevance, are pure, safe, cost saving and having standards with quality specifications thus beneficial to physician and patient3.
REFERENCES
- Partha MP, Mandal B. A Text Book of Homoeopathic Pharmacy. B. Jain Publishers; 2019.
- World Health Organization. Safety issues in the preparation of homeopathic medicines. World Health Organization; 2009.
- Ghate VU, Ghate US. REVIEW OF HOMOEOPATHIC PHARMACY IN THE LIGHT OF QUALITY CONTROL. safety. 2017 Oct 30;2:3.
- Hahnemann S. Organon of medicine. B. Jain publishers; 2005.
- Patel, Dr. (2017). Quality Control & Legal Issues in Homoeopathic Pharmacy in India. 4. 118.
- Sahani MK. Principles and Practice of Homeophatic Pharmacy for Students.
- Banerjee DD. Textbook of homoeopathic pharmacy. B. Jain Publishers; 2023.
- INDIA. The Drugs And Cosmetics Act 1940 along with The Drug And Cosmetics Rules, 1945, CFF-1A, Dilkhush Industrial Istate, G. T. Karnal Road, Delhi 110033: Universal Law Publishing Co. Pvt. Ltd, 86-136
- Dr. Goel S. (2007) Art And Science Of Homoeopathic Pharmacy, 2 nd edition, 8, New Hari Niwas, dattatray Road, Santa Cruz (W), Mumbai 400054: Mind Technologies, 390-422.
- INDIA. Mehera M. L. (2003) GMPS Guide To Good Manufacturing Practices, 3 rd edition, CFF-1A, Dilkhush Industrial Istate, G. T. Karnal Road, Delhi 110033: Universal Law Publishing Co. Pvt. Ltd, 86-136.
- Cook TM. Quality control in the manufacture of homœopathic medicines. British Homeopathic Journal. 1978 Jul;67(03):208-10.
About the Autor: Dr. Anoohhya Uppulurri (BHMS)